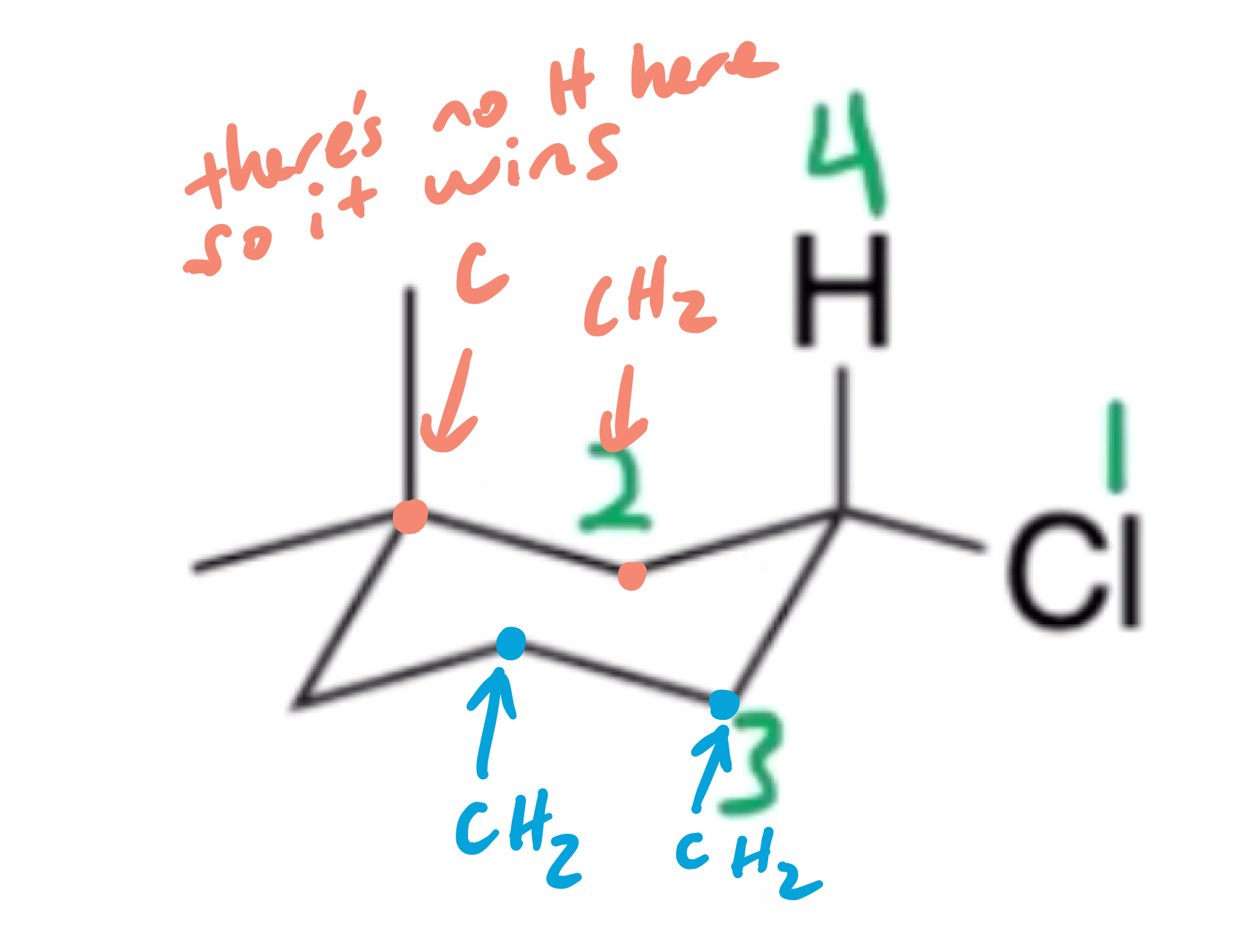
The higher priority group will have less hydrogens, this is why it is numbered this way.
Replied on Lesson: Naming Compounds With Chiral Centers
Replied on Lesson: Resonance and Arrow Pushing
Replied on Lesson: Functional Groups
Replied on Lesson: Functional Groups
Replied on Lesson: Resonance and Arrow Pushing
Replied on Lesson: Naming Aldehydes and Ketones
27 Sep 12:43
Thanks for letting us know Hussein, there's a video on naming multiple functional groups below.
Here's a quick List of priority functional groups from highest to lowest:
Carboxylic acids, Esters, Amides, Aldehydes, Ketones, Alcohols, Thiols, Amines, Ethers, Thioethers, Alkenes, Alkynes, Alkyl Halides
Lesson: Naming Compounds Wi...