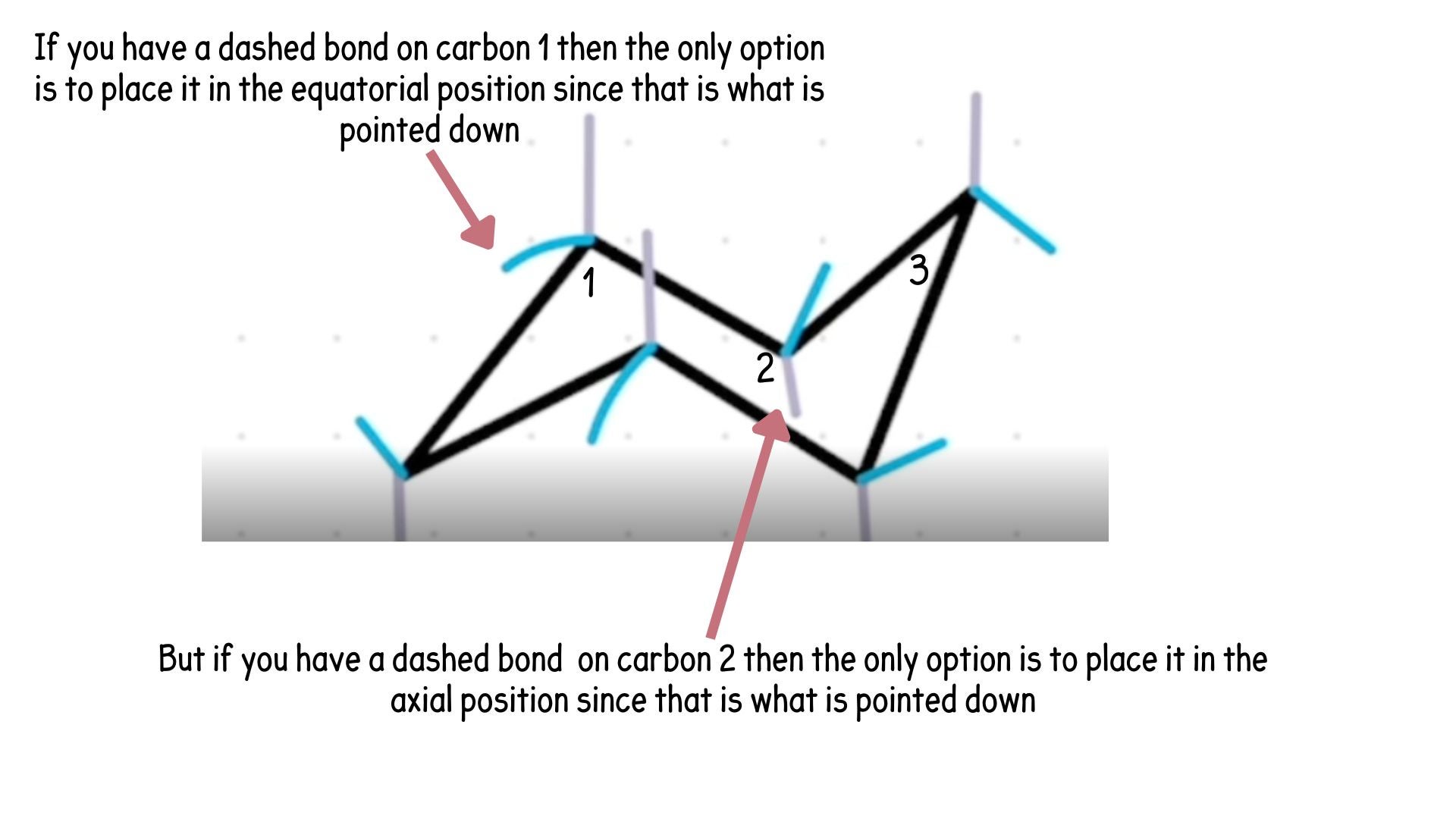
It all depends on the carbon it is placed at, this goes back to 11:30. See below image explanation.
You also are able to number the chair how you would like of course I recommend choosing whatever makes sense for the bond you have. This is why at 29:52 I labelled Carbon 1 in this position because I wanted the bulky group of isopropyl to be in the equatorial position and be pointing up.



Replied on Structure, Bonding, and Properties of Organic Molecules
19 Jun 15:59
If you are talking about the same structure at 22:40, no that is not a valid structure because there aren't enough carbons. The main carbon chain has 6 carbons and 1 carbon sticking out (a substituent), so there is a total of 7 carbons in the structure.
Same with your lewis structure it is missing a carbon.
You are able to place the CH3 on the top and bottom like you have in the lewis structure, that's the same. Just make sure to count your carbons so you don't miss any.